How muscle stem cells keep their nucleus intact to maintain regenerative power
Transcription factor TAF4A preserves nuclei in muscle stem cells and initiates muscle regeneration after injury
Muscle stem cells ensure repair of muscles after injury a person's lifetime. This ability relies on the stability of the cells themselves. Scientists at the Max Planck Institute for Heart and Lung Research in Bad Nauheim have identified a key protein that acts as a guardian of muscle stem cells. The transcription factor TAF4A ensures the stability of the cell nucleus and regulates the balance between the resting and regenerative phases of muscle stem cells. If this factor is missing, the nucleus can lose its shape and even break apart, leading to the random loss of genetic information and disruption of the stem cells’ self-preservation. By keeping the nucleus intact, muscle stem cells stay healthy, rest quietly until needed, and can effectively regenerate muscle after injury. This study provides new insights into muscle stem cell homeostasis and may help to better understand neurodevelopmental disorders, degenerative muscle diseases, and stem cell aging — and perhaps one day prevent them.
Specialized stem cells lie dormant alongside every muscle fiber. These muscle stem cells, also known as satellite cells, ensure that damaged muscle fibers are renewed when injury occurs. To function throughout life, they must be able to both renew themselves and remain in a dormant state until needed. Once activated, they must rapidly repair damaged tissue to restore muscle integrity.
Scientists from the department “Development and Remodeling of the Heart” headed by Director Thomas Braun at the Max Planck Institute for Heart and Lung Research in Bad Nauheim, have now identified that TAF4A, a component of the general transcription machinery, plays a critical role in maintaining the integrity and function of muscle stem cells.
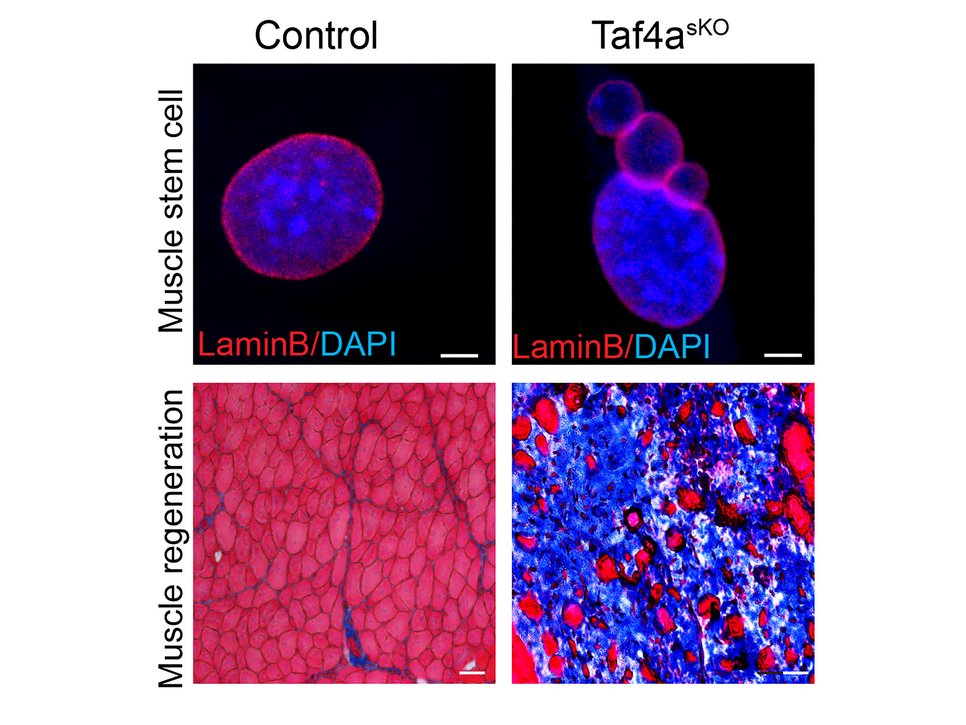
“TAF4A is a transcription factor that controls the expression of an important component of the so-called NSL complex. This complex is responsible for lamin A/C acetylation and the stability of the nuclear lamina - a supportive network inside the cell nucleus,” explains Angelina Georgieva, first author of the study. The Max Planck researchers found that in genetically modified mice where TAF4A was switched off in muscle stem cells, the cells became prematurely activated and their reservoir was rapidly depleted. “Dormancy maintenance during the resting phase no longer functioned in animals without TAF4A, resulting in the depletion of the muscle stem cell pool,” says Georgieva. The absence of TAF4A led to DNA damage, malformed nuclei, and severely impaired muscle regeneration after injury. Instead of new muscle fibers, scar tissue formed.
Mutations in the TAF4A gene are also known to cause TAF4-related neurodevelopmental disorders in humans. The researchers now suspect that skeletal muscle may also be affected in these patients, though symptoms might have been overlooked due to the prominence of neurological complications. Additionally, mutations in lamina genes are known to cause a group of disorders called laminopathies, which affect muscles and other organs and are linked to premature aging and genomic instability. The Max Planck researchers show that absence of TAF4A affects the post-translational modifications of lamin A/C, leading to nuclear abnormalities and genomic instability.
“Ultimately, TAF4A prevents the cascade of molecular events leading to irreversible damage in muscle stem cells,” explains Prof. Thomas Braun. “When this guardian is missing, the cells lose their function and the muscles lose their ability to regenerate.”
“The study offers fresh insight into the molecular mechanisms that protect muscle stem cells required for muscle regeneration and represents a promising starting point for developing new therapeutic strategies for TAF4-related neurodevelopmental disorders, laminopathies, and age-related muscle degeneration,” co-author Xuejun Yuan adds.
