Circadian rhythms in heart metabolism
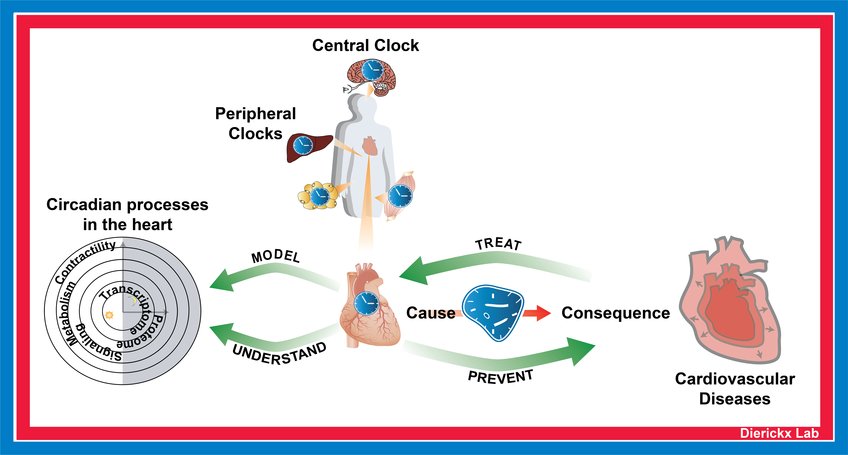
The Dierickx lab is interested in how the circadian clock drives rhythmic processes in the heart. Circadian rhythms coordinate many aspects of behavior and physiology (e.g., fasting/feeding cycles, sleep to wake transitions and body temperature) to be in synchrony with the 24-hour rotation of the earth. In humans, disruption of these rhythms is highly associated with increased risk for cardiovascular disease development. The indispensability of clock proteins in the heart is mechanistically illustrated by our recent findings that REV-ERB loss in cardiomyocytes specifically leads to dilated cardiomyopathy and premature death in mice.
Projects in the lab are centered around an integrated approach combining next-generation sequencing techniques, whole-body physiology analysis in specialized mutant mouse lines and the use of in vitro cardiac models. We aim to develop cardiometabolic insufficiency treatment strategies and will use data from human patients to better understand how deregulation of the clock contributes to heart disease. Investigating how the circadian clock and its intricate connection to rhythmic metabolic programs can be deployed to tackle prevention, diagnosis and treatment of HF is the overarching goal.
We are always on the lookout for passionate people to join our team! Experience with animal models, (stem) cell culture and/or bioinformatic analysis of next-gen data (e.g. single nuclei RNA-seq/ATAC-seq/Cut&Run) would be a great plus.
Informal inquiries are welcome and should be sent to this email address.