Therapeutic key for lung cancer discovered
Reprogramming of certain white blood cells around tumor tissue inhibits tumor growth
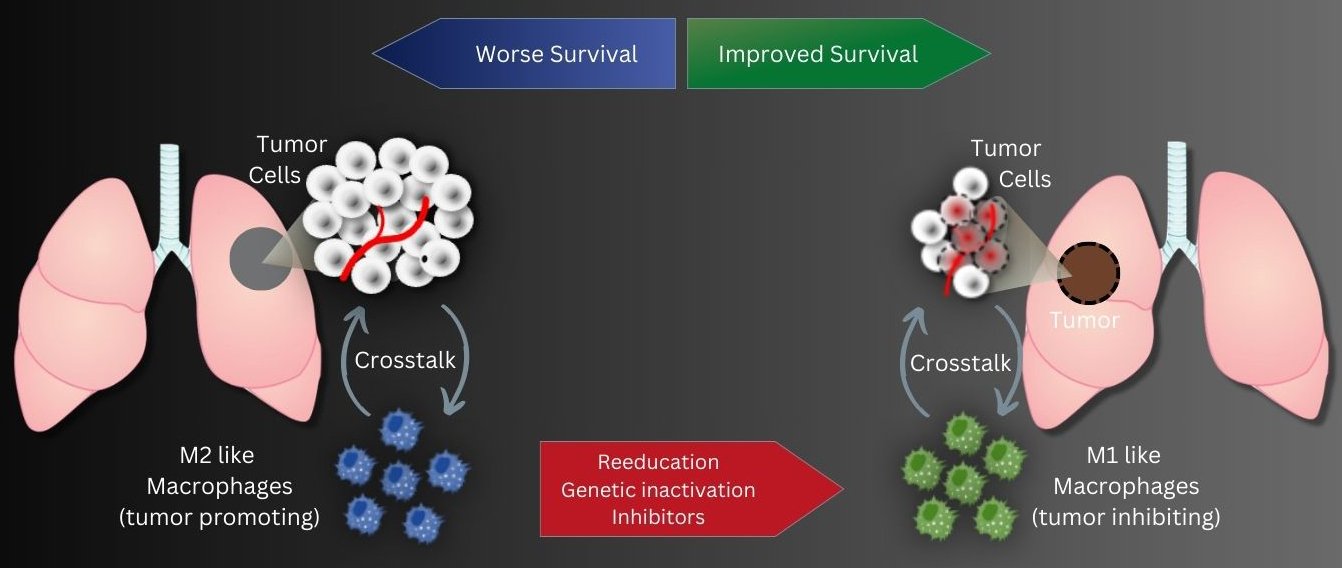
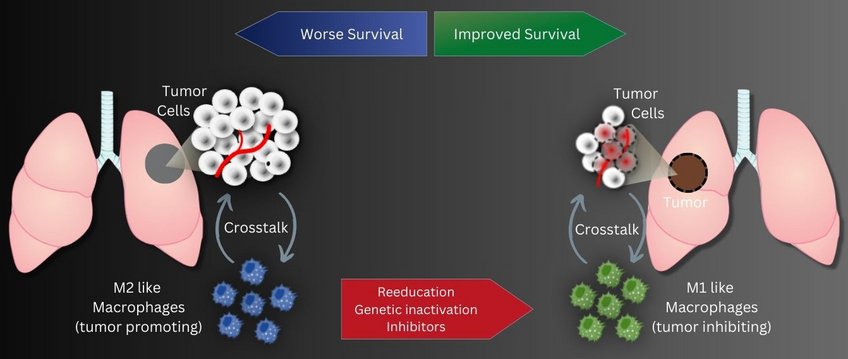
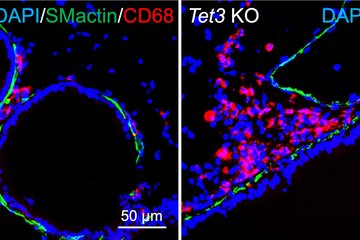
Tumor-associated macrophages, abbreviated TAM, play an important role in the progression of lung cancer. This special group of white blood cells, accumulates in the vicinity of tumor tissue. Previous studies showed that the more cells, especially of the tumor-promoting M2 subtype, are found in the vicinity of the tumor tissue, the higher the probability of an unfavorable course of lung tumor disease. In contrast, a second subtype, M1-TAM, has a more tumor-inhibitory effect.
In two different research approaches, DZL scientists from the Max Planck Institute for Heart and Lung Research in Bad Nauheim and the Justus Liebig University in Giessen now tried to create a tumor-inhibiting environment from a tumor-promoting one by reprogramming tumor-associated macrophages.
"Our strategy aims to reprogram tumor-promoting M2 TAMs into an M1-like anti-tumor cell type using genetic engineering," explains Rajkumar Savai, who led the studies.
To do this, the research group used a special type of RNA called long non-coding RNA (lncRNA). This RNA type influences the transcription process in many cell types. Transcription is the synthesis of RNA from a DNA template. Savai and his colleagues identified an lncRNA called ADPGK-AS1 in M2-TAM. "After we activated the macrophages in the experiment, these cells produced extra ADPGK-AS1. In addition, the RNA shifted from the cytoplasm to the mitochondria, where it affected energy production," Savai said. As a result, ADPGK-AS1 at high levels transformed the cells into an M2-like tumor-promoting cell type. To investigate whether, conversely, lacking ADPGK-AS1 could have an opposite effect, the scientists switched it off by genetically engineering macrophages. "In fact, we found that knocking out ADPGK-AS1 slowed tumor growth in both cell culture and animal experiments," Savai explains."We believe that targeting the production of ADPGK-AS1 in macrophages may be a viable approach to therapy."
The scientists also investigated the influence of epigenetic regulation of tumor-associated macrophages in a second study. The focus was on the enzyme histone deacetylase 2 (HDAC2). Evaluation of biopsies from lung cancer patients revealed a lower survival rate when M2-like macrophages produced particularly high levels of HDAC2. "Here, too, we therefore took the experimental approach of knocking out HDAC2 production in M2 TAMs. In fact, this led to the cells subsequently producing more M1-type marker proteins and fewer M2-type marker proteins," Savai said. In cell culture experiments, this affected tumor cell growth: Silencing HDAC2 in TAMs led to reduced cell division activity in tumor cells. In addition, more tumor cells died. This was confirmed in the mouse model: by pharmacologically inhibiting HDAC2, the resulting switch in macrophage phenotype from M2 to M1 led to delayed tumor growth. "With these two studies, we have succeeded in showing that epigenetic changes in macrophages in the tumor environment can enhance lung tumor growth," Savai said. "An immunosuppressive environment is created that compromises the efficacy of tumor therapies. "Therefore, Savai hopes to have found a therapeutic option with the inhibition of epigenetic regulators such as ADPGK-AS1 and HDAC2. "Immunotherapy targeting these two regulators in lung tumor-associated macrophages may represent a promising new strategy," Savai said.