Epigenetic memory of smooth muscle cells perpetuates chronic asthma
TET3 protects smooth muscle cells from rampant inflammatory responses
Bronchial asthma is the result of chronic inflammation of the airways. In particular, bronchial smooth muscle cells contribute to the disease. Scientists from the Max Planck Institute for Heart and Lung Research in Bad Nauheim have now identified a fundamental mechanism in the mouse model that perpetuates inflammatory reactions in asthma: The reduction of an important modification of the genetic substance DNA (5-hydroxymethylcytosine), triggered by the absence of the enzyme TET3, leads to an escalation of inflammatory reactions in the bronchi. Fittingly, less DNA modification was found in the airways of asthma patients than in healthy individuals. A new therapeutic approach could be based on a drug-induced increase of DNA modifications in asthma patients in order to free the epigenetic memory of smooth muscle cells from the "bad" memories of inflammation.
In bronchial asthma, parts of the airways are narrowed. In addition to various symptoms such as increased mucus production and coughing, patients find it difficult to breathe because of the narrowed bronchi. Whether bronchi dilate or constrict is determined by smooth muscle cells in bronchial tissue. Chronic inflammation in asthma and the associated accumulation of inflammatory cells lead to increased contraction of smooth muscle cells, but also to their proliferation. Chronic inflammation in asthma often persists even after the original trigger, such as an allergic reaction, has long since disappeared. In this context, one speaks of an epigenetic memory of the airways, which perpetuates the disease process. To investigate the molecular and cellular mechanisms of such an epigenetic memory in asthma, scientists from the Max Planck Institute for Heart and Lung Research in Bad Nauheim, Germany, led by Xuejun Yuan and Thomas Braun, have conducted a study to investigate changes in smooth muscle cells in bronchial asthma.
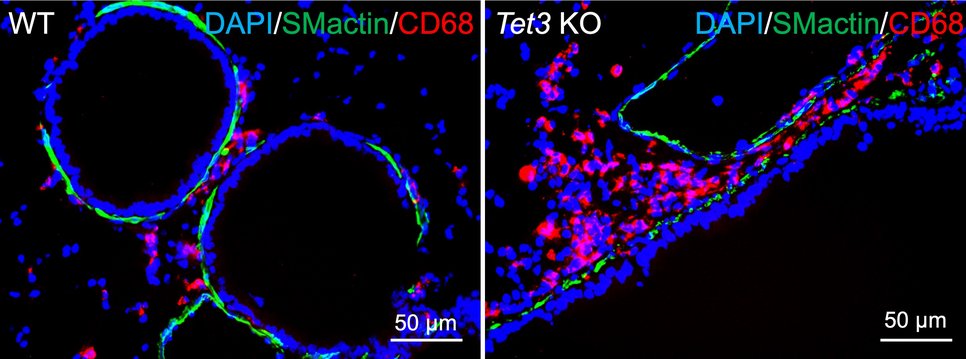
In sample material from asthma patients, the scientists found a greatly reduced proportion of a modified base (5-hydroxymethylcytosine, abbreviated 5-hmC), a component of DNA. 5-hmC is an oxidation product of 5-methylcytosine (5-mC) and was originally thought to be a component of the degradation pathway of 5-mC. However, 5-hmC is now known to have other functions. "Through a reaction known as methylation, genes are usually permanently turned off. This allows a cell to perform its functions correctly," said Fan Wu, first author of the study. "However, the oxidation product of 5-hmC has another role, preventing a faulty start of gene expression. In asthma patients, this process does not work properly. As a consequence, many faulty transcripts are formed." These in turn trigger the inflammatory processes typical of the disease.
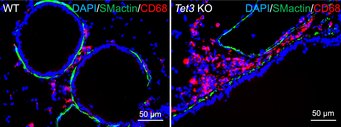
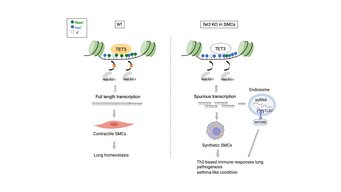
In the animal model, the research team succeeded in analyzing the significance of this observation in more detail. The enzyme TET3 is responsible for the oxidation of 5-mC and thus for the formation of 5-hmC epigenetic DNA methylation in smooth muscle cells. The researchers studied mice specifically lacking the TET3 gene in smooth muscle cells. "Compared to control animals with a functioning TET3 gene, the animals lacking the TET3 gene had much less 5-hmC in airway smooth muscle cells and developed marked inflammation. Furthermore, the number of mucus-forming, so-called Goblet cells increased dramatically. This means that a reduced presence of TET3 in the airways leads to asthma," Wu explains. Comparable to the observation made in asthma patients, there were also other pronounced changes in the bronchial musculature.
In further experiments, the Max Planck researchers investigated the underlying molecular mechanisms and the resulting consequences. They found that 5-mC or TET3 stabilizes the activity of a chromatin-modifying enzyme that prevents gene expression from starting at the wrong site. If gene expression occurs in the wrong place, irregular messenger molecules (mRNAs) are produced that trigger immune responses and strong inflammatory processes. "These inflammatory processes, which also involve massive immigration of immune cells into the lung tissue, provide the basis for the development of asthma-typical symptoms," Wu said.
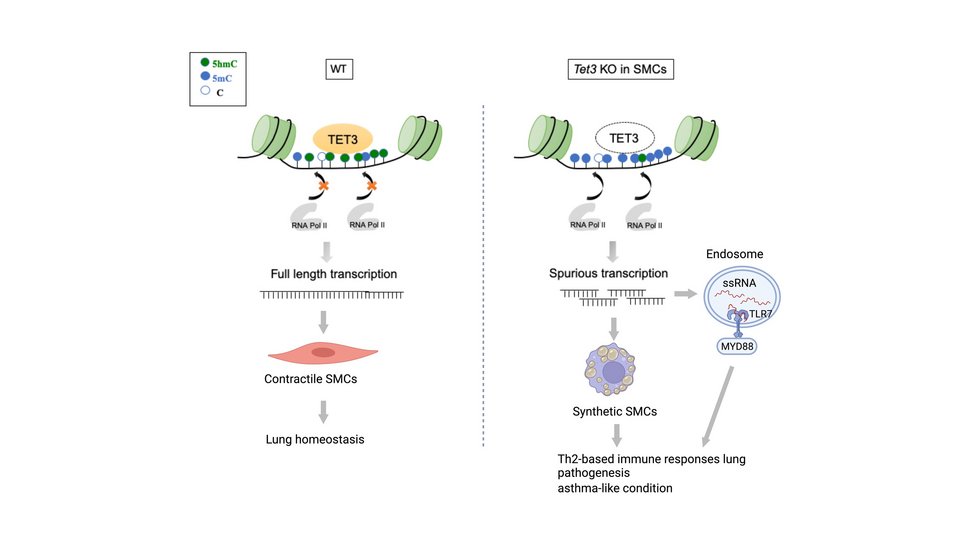
According to Thomas Braun, director at the Max Planck Institute in Bad Nauheim, Germany, the study provides an explanation for why asthma can become chronic in patients even when the original asthma-causing allergen is no longer present: "The insufficient modification of DNA in smooth muscle cells is probably a memory of epigenetic memory. This causes the inflammatory processes that once occurred to be relived over and over again." The consequence of this is a persistence of the inflammatory processes and thereby asthma symptomatology. With this study, the scientists hope to have found an approach for the development of new therapy to break the vicious circle of chronic inflammation. Such an approach could be based on increasing the activity of the TET3 enzyme and thus of 5-hmC in smooth muscle cells and thus erasing the epigenetic memory.
MH/TB