MRI: Cardiac Imaging
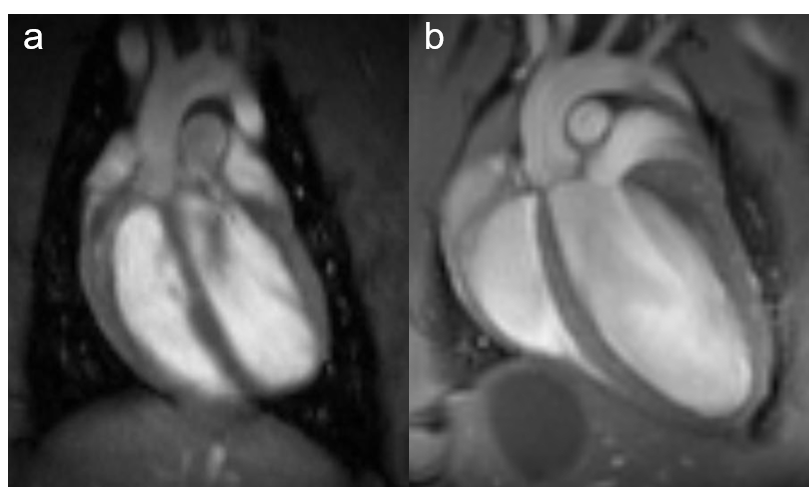
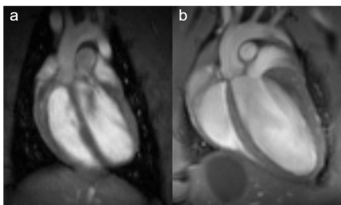
Fig. 1: Rat and mouse heart MRI. 4-chamber-views of a rat (a), upper panel and mouse heart (b), lower panel are displayed. Beside the lumen of the left and right chamber, the left and right atria, the myocardium and the up-going vessels are visible. For rat a 72 mm volume transmit-only together with 20 mm recieve-only planar surface coil, both room temperature, and for mouse the 72 mm volume transmit-only and the cryogenic 4 element 1H array receive-only coil were used. Intragate Flash (Fast Low Angle Shot) sequence for rat (repetition time: 6.2 ms; echo time: 1.3 ms; oversampling:150; flip angle: 10 deg; slice thickness: 1.0 mm; FOV: 45x45 mm and matrix: 128x128 .Intragate UTE (Ultra Short Echo Time) for mouse: repetition time: 8.0 ms; echo time: 0.36 ms; oversampling: 100; flip angle: 15 deg; slice thickness: 1.0 mm; FOV: 22x22 mm and matrix: 128x128.
MRI is a well-established modality for imaging of the cardiovascular system in rat and mice. Beside determination of tissue, organ or tumor sizes, our main focus lies on monitoring changes over time after treatment of transgenic mice. One of the best-known forms of dynamic MRI experiments is the functional MRI, typically monitoring changes in blood flow. In the recent years MRI has become the standard for the quantitative evaluation of cardiac function, masses, and infarct size. Wall motion and strain analysis are used to display myocardial dysfunction. To obtain information on the morphology and functional parameters of rat and transgenic mice heart, we established standard protocols that enable us to rapidly acquire high quality images.
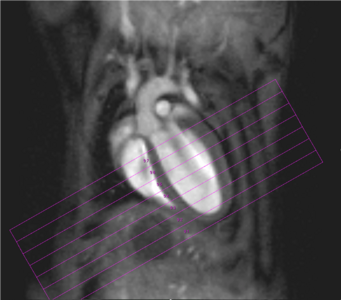
Physiological gating is required to minimize motional influence of the beating heart and respiration on the MR experiment but also to synchronize the imaging sequence to the cardiac cycle. Beside ECG electrodes and wires, a respiration sensor as well as tubings for inhalation and anaesthesia have to be kept within the probe head. In all measurements body temperature of the examined animal has to be maintained via thermostatically regulated water flow system. Currently it is possible to carry out all experiments necessary for the determination of the functional parameters of rat or mouse ventricles, like end-diastolic, end-systolic, and stroke volumes, ejection fraction, cardiac output, ventricular mass and wall-thickness within less than 45 minutes (Fig. 2).
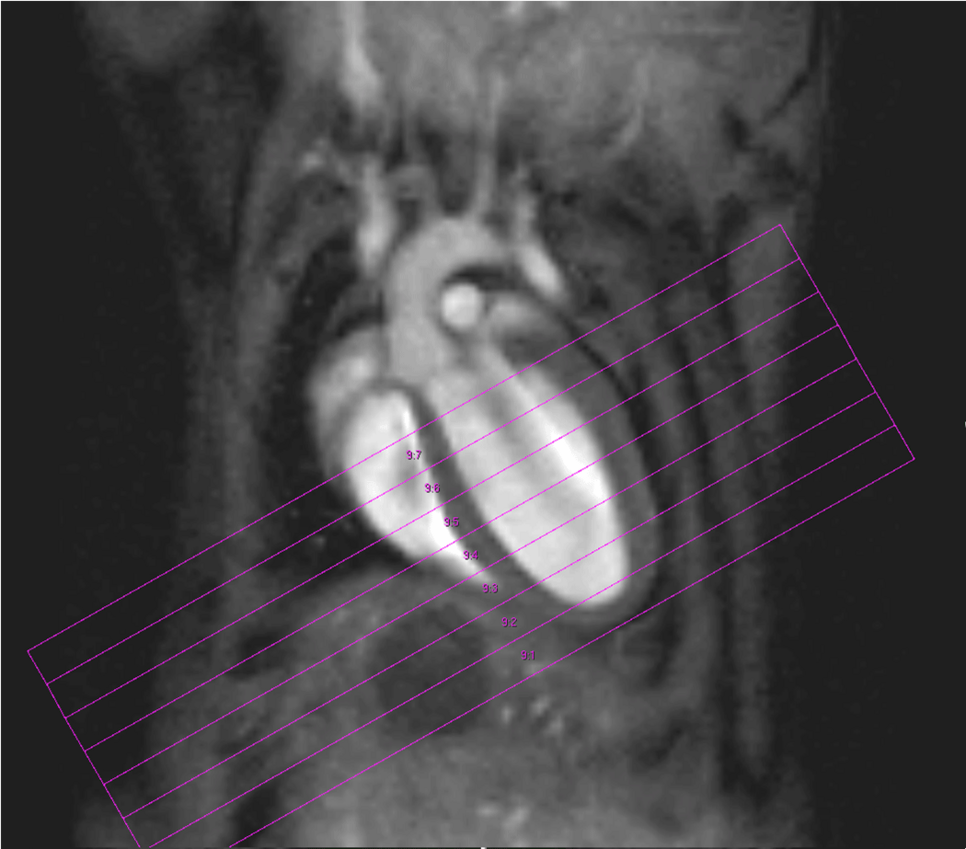
Fig. 2: Mouse heart MRI. In the upper panel the endsystolic (a) and enddiastolic (b) phase of a mouse heart in the 4-chamber-view is displayed. In red: position of the 7 slices, orthogonal to the septum (1.0 mm thick, no gap). In the lower panel in (c) the endsystolic and in (d) the enddiastolic phase of an axial midventricular slice is shown. Note the papillary muscle.
This is also due to the fact, that we use retrospective gating technique: Cardiac and respiratory cycles are detected by a navigator signal, and therefore no triggering hardware is required. The so-called “self-gating” IntragateTM Tool (Bruker BioSpin, Ettlingen, Germany) provides a steady state condition which avoids the flashing effects common to conventional ECG triggering and respiratory gating. Especially for our newt the renouncement of electrodes fixated on the tiny legs is much more comfortable for animal and investigator. The self-gating method of Intragate additionally uses intrinsically a kind of segmentation – several echoes during one heart cycle are utilized for the same image – significantly reducing the measurement time for the same SNR compared with the classical methods triggered by the ECG-signal (Fig. 3).
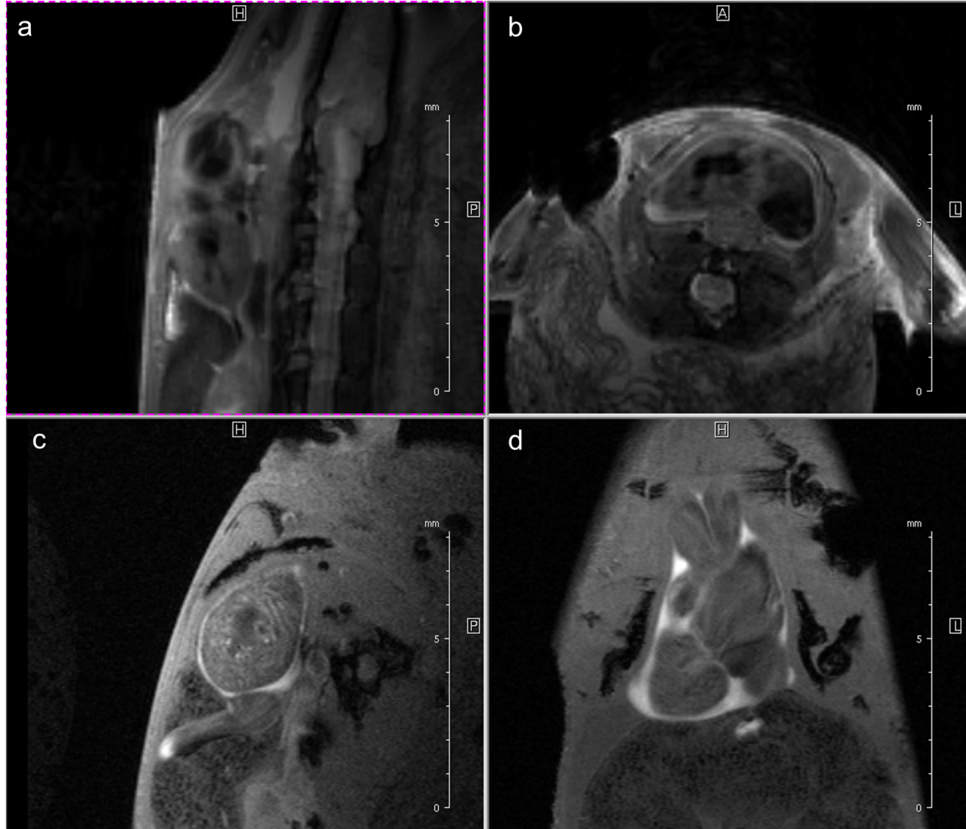
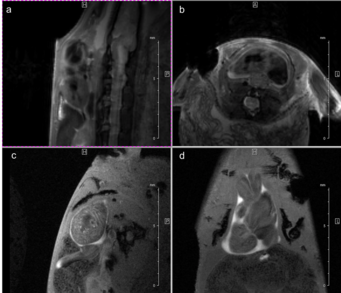
Fig. 3: Newt heart MRI. In the upper panel two localizer scans (RARE method (rapid acquisition with relaxation enhancement) with a repetition time of 2500 ms, echo time of 36.7 ms, slice thickness/interslice distance: 0.50/0.50 mm, field of view 2.50x2.50 cm2 and a matrix of 256/256 (a)) are shown: two 2-chamber views (a,b). In the lower panel an image of the ventricle in axial (c) and coronal (d) orientation is presented. We imaged the animal with an Intragate Flash (Fast Low Angle Shot) sequence: repetition time: 5.6 ms; echo time: 2.9 ms; oversampling: 150 (c) or 300 (d); flip angle: 10 deg; slice thickness/interslice distance: 0.3/0.3 mm; FOV: 15x15 mm and matrix: 256/256 (c) or 128/128 (d) using the 72 mm volume transmit-only room temperature and the cryogenic 4 element 1H array receive-only coil.
For our latest project, cine cardiac MR imaging of living adult zebrafish, the retrospective gating technique (IntragateTM Tool) is mandatory. The small size of the zebrafish heart, the ventricle comprises around 2 mm, and the fact that we deal with a fish makes, MRI quite challenging. Being able to use a cryogenically cooled 1H array coil, which gives us a much higher resolution and a better signal to noise ratio compared to a room temperature coil, we are able to image adult zebrafish hearts. The biggest challenge is the supply of the fish with oxygen during the measurements. We are using two cradle set-ups momentary: A chamber with water flow (Fig. 4a), which leads to a prolongation of measurement time, a more constant heart beat and less spontaneous movement of the anesthetized fish; but with this method the natural gill movement -which frequency is close to the frequency of the heart of the fish- is difficult to be separated from the heart movement.
Another possibility is to position the fish in a chamber without floating water so gill movement will stop, but due to the unfavourable situation with oxygen supply, only short measurement time is possible.