Cardiac regeneration becomes possible through reprogramming of cell metabolism
Reprogramming of energy metabolism activates division activity of cardiac muscle cells and restores cardiac function after infarction.
After birth, the human heart loses its regenerative capacity almost completely. Damage to the heart muscle, e.g. due to a heart attack, therefore usually leads to a permanent loss of function in adults. Scientists from the Max Planck Institute for Heart and Lung Research have now shown for the first time in an animal model that a change in the energy metabolism of heart muscle cells enables heart regeneration. In the mouse model, heart function could thus be restored to a large extent after a heart attack. The study, published in the journal Nature, is groundbreaking and could enable completely new therapeutic approaches.
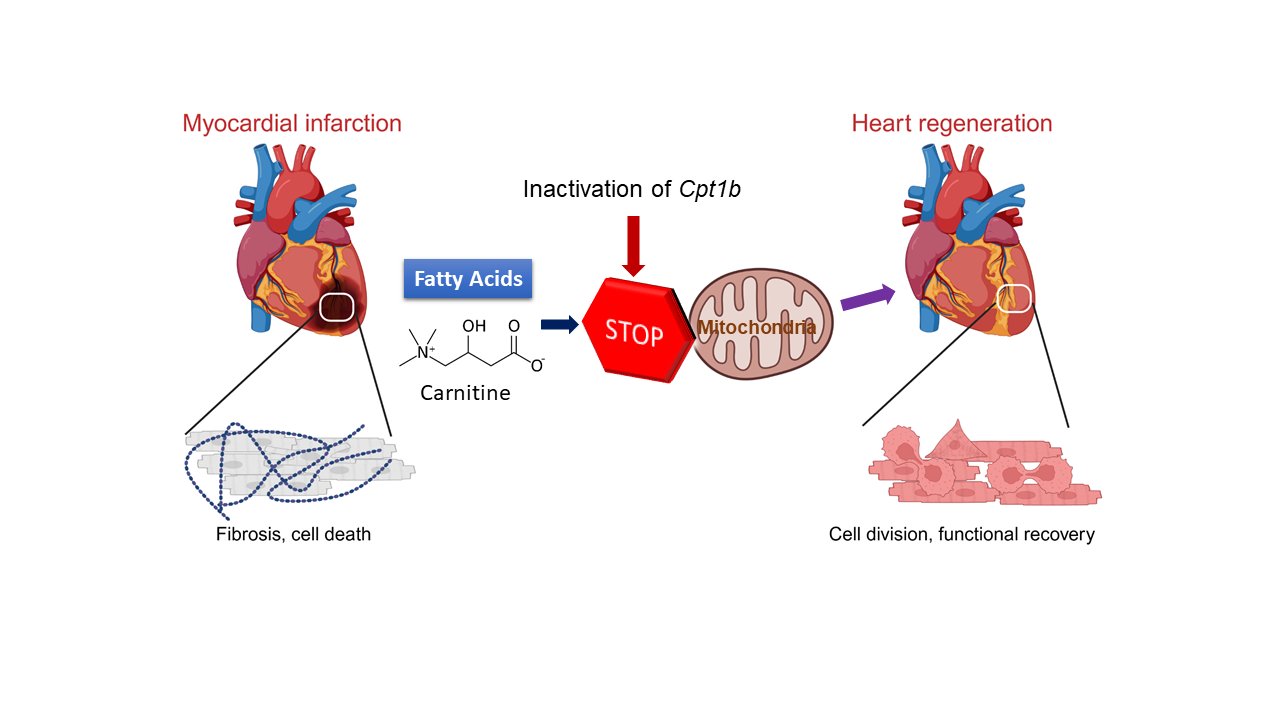
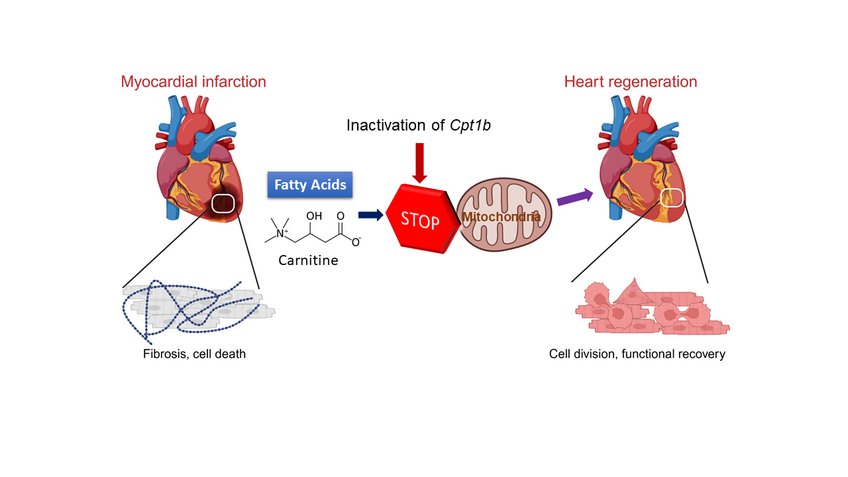
Shortly after birth, the human heart largely loses its ability to repair itself in the event of damage. Injuries or diseases of the heart muscle therefore lead to permanent damage and a reduction in heart function. The loss of regenerative capacity is due, among other things, to the loss of the ability of heart muscle cells to divide after birth. This is accompanied by a fundamental change in the energy metabolism of the heart cells: Instead of obtaining energy from sugars, which is known as glycolysis, the heart muscle cells now obtain their energy largely from fats. This form of energy production is known as fatty acid oxidation.
The research group led by Thomas Braun, Director at the Max Planck Institute for Heart and Lung Research in Bad Nauheim, Germany, started looking for new methods to promote heart regeneration: "It is known that animal species capable of regenerating their hearts primarily use sugars and glycolysis as fuel for heart muscle cells. The human heart also primarily uses glycolysis in early stages of development, but then switches to fatty acid oxidation because it can produce more energy," explain Xiang Li and Xuejun Yuan, authors of the study. "With the switch in energy production after birth, the activity of many genes changes and cell division activity is lost. Individual metabolites from energy production also have important functions for the activity of enzymes that regulate gene activities. We therefore hoped to trigger changes in gene activities by reprogramming energy metabolism to turn cell division ability back on in cardiac muscle cells."
To do this, the Max Planck researchers first inactivated a gene called Cpt1b, which is essential for fatty acid oxidation, in mice. "We then observed that hearts in these mice started to grow again," Li explains. Over the course of the experiment, cell numbers in the hearts of these mice nearly doubled.
In the next step, the Bad Nauheim researchers triggered heart attacks in mice in which Cpt1b was switched off. In the chosen approach, a phase of lack of blood flow to the heart is followed by a reperfusion phase, in which the heart is flushed again with oxygenated blood. "This model is comparable to a cardiac patient whose heart is treated with the insertion of a stent due to an occlusion of the coronary arteries," Yuan explains. The effect was impressive: otherwise common scars in the heart muscle were barely noticeable after weeks, and heart function (contractility) in animals without Cpt1b almost returned to pre-infarction levels.
In further studies, the scientists were able to decipher the underlying mechanism: "In heart muscle cells of the mice with the inactivated gene, we found a twenty-fold increased level of alpha-ketoglutarate. The high level of this metabolite leads to a significant increase in the activity of the enzyme KDM5," Braun explains. This enzyme is a so-called histone demethylase, which removes methyl groups from histones and thus reduces the activity of various genes. The change in gene activity causes cardiac muscle cells to become immature and thus regain the ability to regenerate.
Braun sees the study as a real breakthrough: "By reprogramming the metabolism, we double the number of heart muscle cells, and after an infarction, heart function is almost completely restored."
In addition, it is possible in principle to pharmacologically block the activity of the enzyme CPT1B -the gene product of Cpt1b. The development of an inhibitor that can be used to affect the activity of the CPT1B enzyme is the next step on the road to developing a therapy that may eventually be used in humans. However, Yuan and Braun emphasize, "We still have a long way to go before reliable treatments in humans become possible. The implementation of new findings from basic research is lengthy and expensive and is often accompanied by many unexpected problems. Nevertheless, we are confident that we will be able to therapeutically stimulate the regenerative capacity of the heart in the future."