Protein in blood vessels puts tumour cells into deep sleep
Membrane protein PEAR1 prevents the development of metastases
Some cancer patients develop metastases again after several tumour-free years. This is often caused by individual tumour cells that are in a dormant state in tissue near small blood vessels. Suddenly, these cells undergo cell division again and form metastases. Scientists at the Max Planck Institute for Heart and Lung Research have now analysed more than 500 genes in a screening process. In doing so, they identified a gene whose gene product, known as PEAR1, is located in the membrane of blood vessel cells and is responsible for the dormant state of the tumour cells. If the protein is missing, the tumour cells begin to divide again.
Thanks to medical advances, the chances of recovery from many types of cancer have improved. However, some patients do not die from the primary tumour, but from late-onset metastases – often many years after successful treatment. A problem that remains largely unsolved is that metastatic tumour cells, after spreading over many years, have become established in other organs, but initially remain inactive there. This phenomenon is known as tumour dormancy. Some of these ‘sleeping’ (‘dormant’) tumour cells can become active again much later and start dividing once more. Metastases then develop from these individual tumour cells. This phenomenon is clinically relevant in breast cancer, prostate cancer and melanoma, for example.
The mechanisms underlying this tumour cell dormancy and how dormant tumour cells are reactivated have been largely unknown until now. Scientists from the department of Stefan Offermanns, Director at the Max Planck Institute for Heart and Lung Research in Bad Nauheim, have now discovered a protein that prevents tumour cells from re-entering cell division.
‘It is known that dormant tumour cells leave the normal cell cycle and enter the so-called G0 phase, in which they no longer divide. Dormant tumour cells are found particularly in the walls of small blood vessels in various organs and are in close contact with the cells of the inner vessel wall, the endothelial cells,’
explains Kenneth A. Roquid, first author of the study. The Bad Nauheim scientists suspected that the microenvironment of these niches inactivates the tumour cells, whereas a disturbance of this environment, e.g. due to inflammation or injury, triggers renewed growth of the tumour cells.
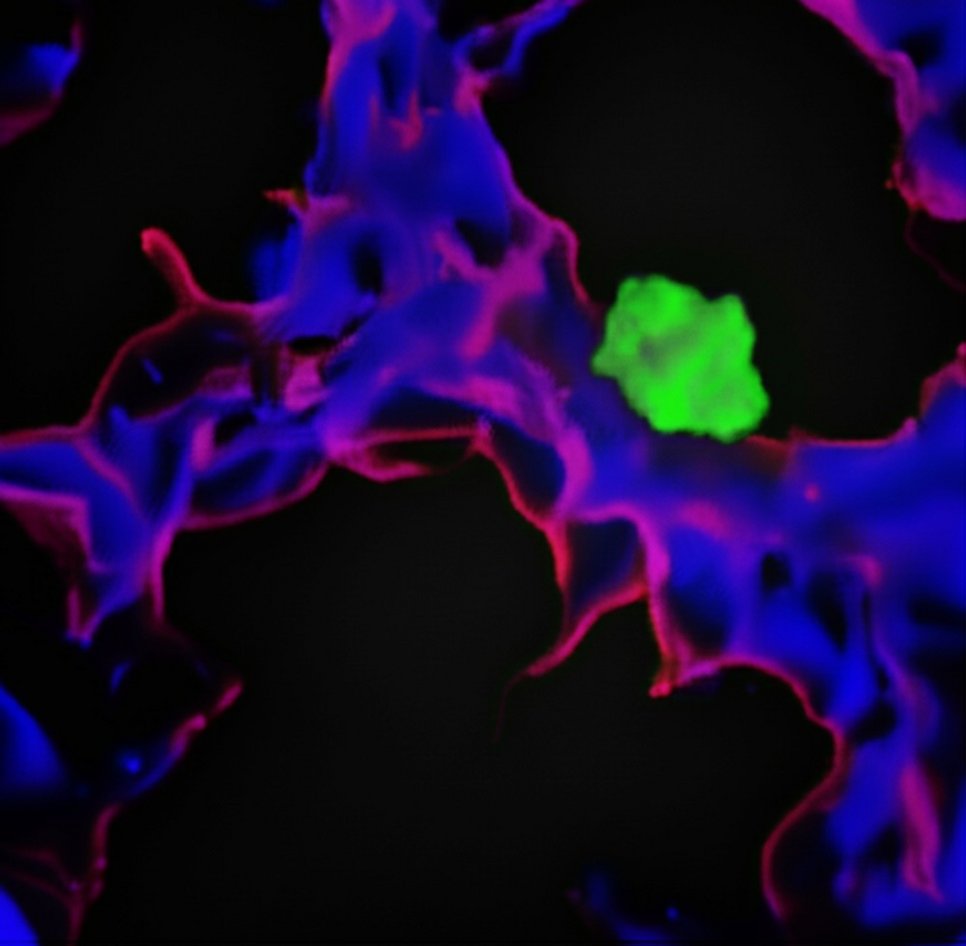
‘In the first step, we developed a cell culture test system in which we could cultivate endothelial cells and dormant tumour cells together. We then systematically switched off over 500 genes in endothelial cells,’ said Roquid. These genes encoded proteins on the surface of endothelial cells or proteins released by endothelial cells. ‘In our test system, we then investigated whether switching off a gene caused the tumour cells to lose their dormancy,’ Roquid continued. Ultimately, the Max Planck researchers identified PEAR1, a protein located in the plasma membrane of endothelial cells that keeps tumour cells dormant.
In the next step, Offermanns' team tested the discovery in living organisms: ‘In mice in which the expression of PEAR1 was selectively suppressed in endothelial cells, we observed significantly more metastases than in the control group,’ co-author Adriana Vucetic summarises the findings. She explains the mechanism as follows: ‘Two other proteins, known as LOXL2 and CTSD, normally prevent tumour cells from going dormant. When LOXL2 and CTSD are reduced, dormancy increases and fewer metastases form in the mice,’ says Vucetic. The Bad Nauheim scientists have now shown that PEAR1 binds to these two proteins, inactivates them and thus promotes tumour dormancy. In the absence of PEAR1, LOXL2 and CTSD are active, resulting in more metastases.
"Our results show that the surface protein PEAR1 ensures a stable microenvironment in the vicinity of small blood vessels. In this way, it plays a crucial role in keeping tumour cells in a dormant state,‘
explains Offermanns.
’In the long term, this mechanism could open up new possibilities for preventing late-stage metastases."
However, before clinical application, the Bad Nauheim researchers believe it is necessary to decipher further components of this tumour dormancy-promoting microenvironment in order to further improve our fundamental understanding.
