G-protein-coupled receptors
Project leaders: Dr. M. Wessam Alnouri, Dr. Remy Bonnavion
PhD Students: Mira Lettmann, Mallika Rani Prusty
Technical Assistant: Kathrin Heil
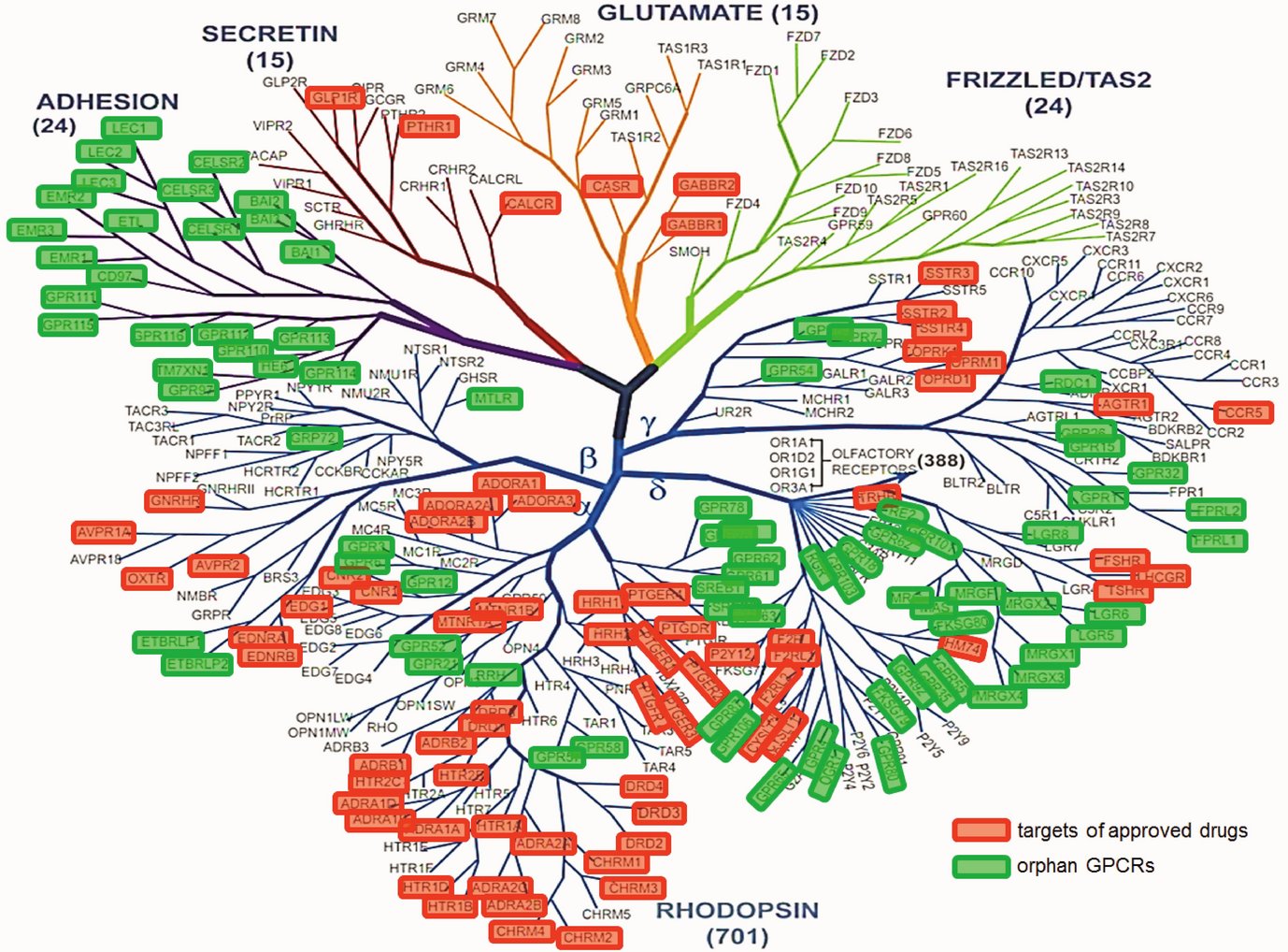
G-protein-coupled receptors (GPCRs) are the largest receptor family in mammals, with about 800 receptors encoded in the human genome. Almost half of the receptors are olfactory receptors, whereas the remaining receptors respond to a wide spectrum of different ligands. For about 150 GPCRs no ligand has so far been described, making them “orphan GPCRs”. Every cell of the body expresses at least 15 to 20 GPCRs, which are involved in the regulation of all body functions. Their high ligand selectivity and their central and specific role in the modulation of physiological and pathophysiological processes make GPCRs ideal drug targets, and about a third of all approved drugs act through GPCRs.
In the past, we have identified new receptors activated by metabolites such as lactate, ketone bodies and free fatty acids (Tunaru et al., 2003; Ahmed et al., 2010; Offermanns, 2014; Blad et al., 2012), receptors binding chemokines (Le Mercier et al., 2021) or receptors activated by specialized pro resolving lipid mediators (SPMs) (Alnouri et al., 2024). Using siRNA-based approaches, we have identified new ligand receptor pairs (Tunaru et al., 2012). Employing complex mouse genetics, we have been able to elucidate the role of several of these receptors in the pharmacological effects of drugs (Hanson et al., 2010; Lukasova et al., 2011), in the regulation of insulin secretion (Tang et al., 2015; Tunaru et al., 2018, Chen et al., 2025), in endothelial flow sensing and blood pressure regulation (Wang et al., 2015; Iring et al., 2019) or in the regulation of vascular insulin sensitivity (Cho et al., 2025). Current work mainly focuses on orphan GPCRs and aims at the identification of new physiological GPCR ligands mainly in the areas of the cardiovascular system, metabolism and cancer.
Literature
- Chen X, Shao J, Brandenburger I, Qian W, Hahnefeld L, Bonnavion R, Cho H, Wang S, Hidalgo J, Wettschureck N, Geisslinger G, Gurke R, Wang Z, Offermanns S. (2025) FFAR4-mediated IL-6 release from islet macrophages promotes insulin secretion and is compromised in type-2 diabetes. Nat. Commun. (in press)
- Cho H, Lai CC, Bonnavion R, Alnouri MW, Wang S, Roquid KA, Kawase H, Campos D, Chen M, Weinstein LS, Martínez A, Looso M, Sanda M, Offermanns S. (2025) Endothelial insulin resistance induced by adrenomedullin mediates obesity-associated diabetes. Science. 387, 674-682.
- Alnouri MW, Roquid KA, Bonnavion R, Cho H, Heering J, Kwon J, Jäger Y, Wang S, Günther S, Wettschureck N, Geisslinger G, Gurke R, Müller CE, Proschak E, Offermanns S. (2024) SPMs exert anti-inflammatory and pro-resolving effects through positive allosteric modulation of the prostaglandin EP4 receptor. Proc Natl Acad Sci U S A. 121, e2407130121.
- Le Mercier A, Bonnavion R, Yu W, Alnouri MW, Ramas S, Zhang Y, Jäger Y, Roquid KA, Jeong HW, Sivaraj KK, Cho H, Chen X, Strilic B, Sijmonsma T, Ralf Adams R, Schroeder T, Rieger MA, Offermanns S (2021). GPR182 is an endothelium-specific atypical chemokine receptor that maintains hematopoietic stem cell homeostasis. Proc. Natl. Acad. Sci. U.S.A. 118, e2021596118
- Iring A, Jin YJ, Albarrán-Juárez J, Siragusa M, Wang S, Dancs PT, Nakayama A, Tonack S, Chen M, Künne C, Sokol AM, Günther S, Martínez A, Fleming I, Wettschureck N, Graumann J, Weinstein LS, Offermanns S (2019). Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Invest. 130: 2775-2791
- Tunaru S, Bonnavion R, Brandenburger I, Preussner J, Thomas D, Scholich K, Offermanns S (2018). 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat. Commun. 9: 177
- Wang S, Iring A, Strilic B, Albarrán Juárez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Müller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S (2015). P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J. Clin. Invest. 125: 3077-3086
- Offermanns S (2014). Free Fatty Acid (FFA) and Hydroxy Carboxylic Acid (HCA) Receptors. Annu. Rev. Pharmacol. Toxicol. 54: 407-434
- Tunaru S, Althoff TF, Nusing RM, Diener M, Offermanns S (2012). Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc. Natl. Acad. Sci. U. S. A. 109: 9179-9184
- Blad CC, Tang C, Offermanns S (2012). G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug. Discov. 11: 601-619
- Lukasova M, Malaval C, Gille A, Kero J, Offermanns S (2011). Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Invest. 121: 1163-1173
- Ahmed K, Tunaru S, Tang C, Müller M, Gille A, Sassmann A, Hanson J, and Offermanns S (2010). An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 11: 311-319
- Hanson J, Gille A, Zwykiel S, Lukasova M, Clausen BE, Ahmed K, Tunaru S, Wirth A, Offermanns S (2010). Keratinocytes express GPR109A and mediate epidermal effects of nicotinic acid and mono-Methyl fumarate via COX-2-dependent prostanoid formation J. Clin. Invest. 120: 2910-2919
- Tunaru, S., Kero, J., Schaub, A., Wufka, C., Blaukat, A., Pfeffer, K., and Offermanns, S. 2003. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9: 352-355