Research
Dr. Savai's lab aims to understand how the lung microenvironmental niche drives tumor initiation and progression and, most importantly, to identify specific targets that will interact with or inhibit the tumor niche's maintenance and functional competence. The long-term goal of Dr. Savai´s lab is to translate tumor microenvironment-based therapeutic approaches into proof-of-concept clinical trials in lung cancer.
Lung cancer is the leading cause of cancer deaths worldwide. Although early-stage lung cancer can be treated with radical interventions or standard cytotoxic chemotherapy regimens, more than 70% of patients relapse and die, primarily due to metastatic progression. Moreover, most patients with stage IV cancer die within 18 months of diagnosis. Although recent clinical trials with immune checkpoint blockade therapy have shown an unprecedented durable response in patients with a variety of cancer, only a subset of patients achieves a stable state of cancer remission. Thus, further in-depth analysis of the molecular mechanisms underlying lung carcinogenesis is mandatory to identify novel molecular targets that may lead to the development of effective targeted therapies. Changes in the local lung microenvironment (niche) of transformed cells accompany malignant growth initiation. In response to cancer cell growth, the niche will evolve and modify its composition to create a complex network that supports the tumorigenic behavior and plasticity required for cancer cell growth, resistance to therapies, recurrence, and metastasis. Therefore, deeper knowledge of the niche establishment and evolution is needed to understand the complexity of cancer and to design better treatments.
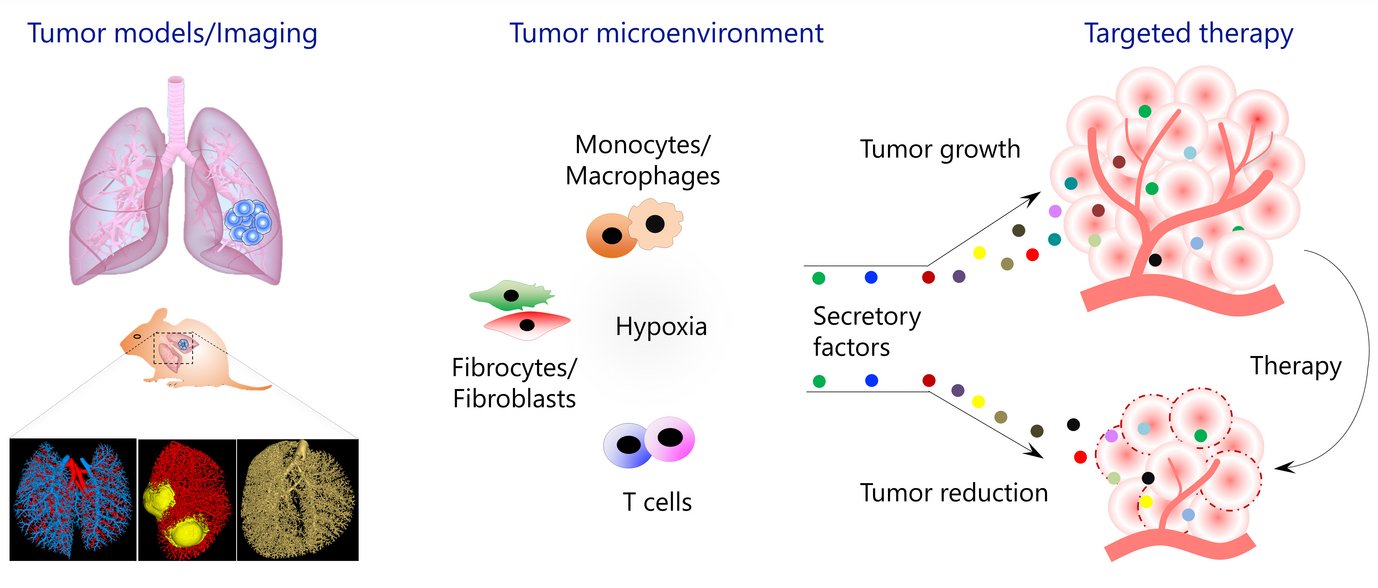
Figure 1: Overview of research topics addressed in Savai group
For the past several years, the Savai Lab is interested in several aspects of lung cancer, especially in the characterization of immune/inflammatory/mesenchymal cells in the tumor microenvironment and delineating the roles of monocytes/macrophages, fibrocytes/fibroblasts, and T cell subpopulations in lung cancer progression and metastasis. To investigate the above, Savai Lab established several lung tumor mouse models (KRas, KRas/p53, and cRaf transgenic lung tumor models as well as primary and metastatic lung tumor models) and developed small animal lung tumor imaging techniques (micro-computed tomography and flat-panel volumetric computed tomography) to detect and monitor lung tumor growth and vascularization. Based on preliminary data of genome-wide profiling of human and mouse microenvironmental cells, the Professorship for Lung Microenvironmental Niche in Cancerogenesis focuses on the following major objectives:
- Understanding the spatiotemporal architecture of the lung microenvironmental niche in cancerogenesis
- Identifying prognostic monocyte/macrophage, fibrocytes/fibroblast, and T cell subpopulation phenotypes using multiplex fluorescence immunohistochemistry (PhenOptics)
2. Identifying and characterizing macrophage heterogeneity in the lung tumor niche
- Identifying and functionally characterizing macrophage subpopulations/heterogeneity by employing integrative single-cell omics and in situ sequencing
- Performing integrated epigenomic and chromatin remodeling analyses of lung-tumor-associated macrophages
- Evaluating novel approaches of re-education/reprogramming of macrophages to treat lung cancer
3. Delineating the role of fibrocyte subpopulations in lung tumor progression and metastasis, with the aim to identify novel selective intervention strategies
4. Rewiring metabolism/signaling as an approach to inhibit the differentiation of macrophages/fibrocytes and form a cancer-supportive niche
5. Establishing new mouse lung tumor models, novel non-invasive imaging techniques, and novel human ex vivo lung cancer models (viable human lung [cancer] slices)
6. Analysis of lung cancer–lung disease crosstalk
- Investigating lung cancer-associated pulmonary hypertension to elucidate the role of microenvironmental/perivascular inflammation based on tumor cell-immune cell crosstalk
- Evaluating the transition of idiopathic pulmonary fibrosis to lung cancer to determine the role of matrix composition and microenvironmental immunological processes